

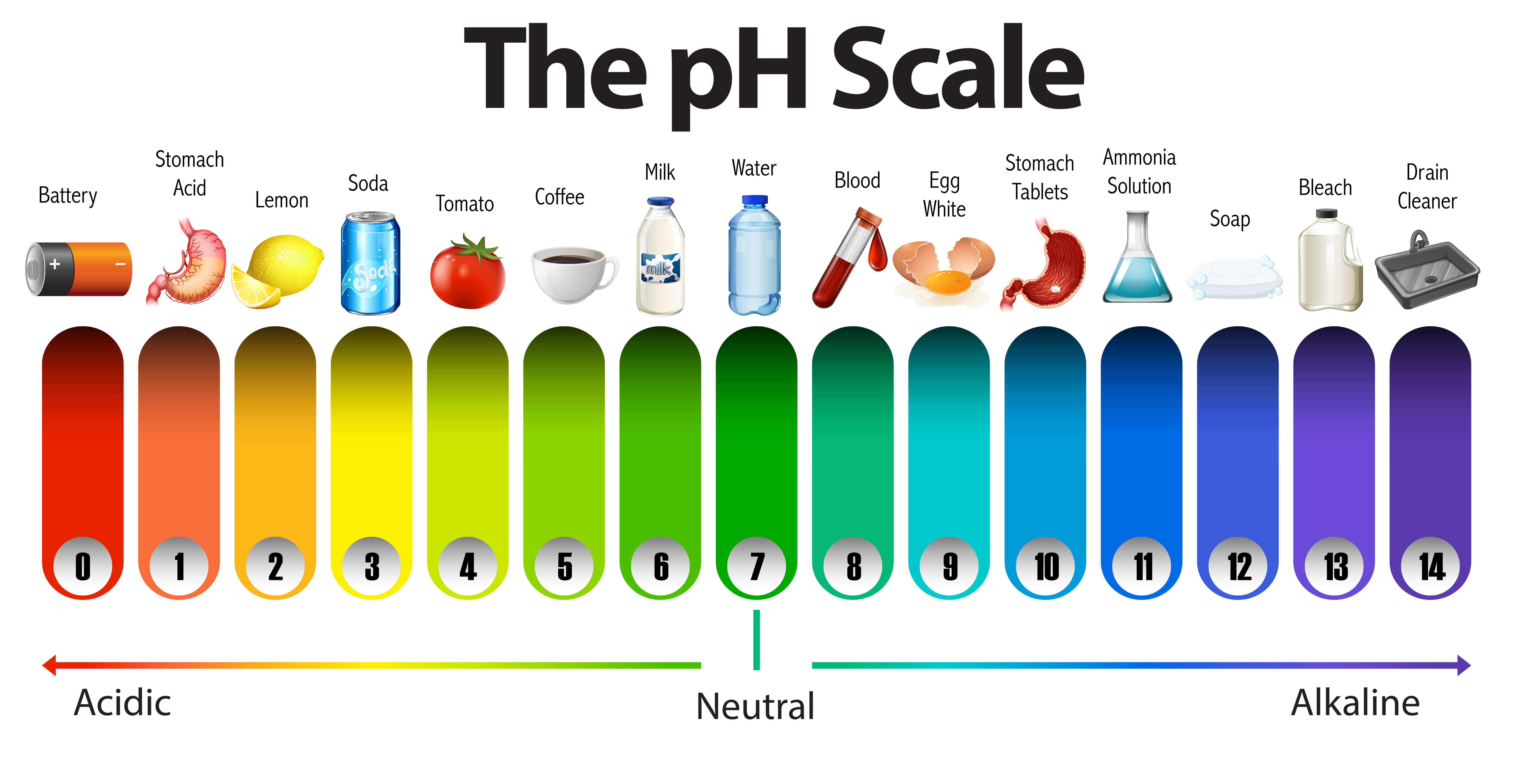
pH is a measure of how acidic or basic a substance is. It is a scale from 0 to 14, with 7 being neutral. Substances with a pH less than 7 are acidic, and substances with a pH greater than 7 are basic.
Acidic substances taste sour, like lemon juice or vinegar. Basic substances taste bitter, like baking soda or soap.
pH is important because it affects the way that substances react with each other. For example, acids can react with bases to form salts. pH also affects the way that living things function. For example, the pH of the blood in humans is very carefully controlled.
The pH of the soil is important for plant growth.
Noun:
Adjective:
The word "pH" is an abbreviation of the German word "Potenz von Hydrogen" (power of hydrogen). The word "Potenz" means "power" and the word "Hydrogen" refers to the hydrogen ion, which is the ion that determines the acidity or alkalinity of a solution.
The word "pH" was first used in 1909 by the Danish chemist Søren Peter Lauritz Sørensen. Sørensen developed the pH scale to measure the acidity or alkalinity of a solution. The pH scale is a logarithmic scale, which means that each unit change on the scale represents a tenfold change in acidity or alkalinity.
What does the pH measure?